40 fda structured product labels
dailymed.nlm.nih.gov › dailymed › fda-drug-guidanceDailyMed - FDA Resources: SPL, Other Prescription Drug ... Structured Product Labeling (SPL) is the standard format for electronic submission of the content of labeling. For SPL resources (including industry data standards for SPL), see FDA's SPL Resources page and the "Structured Product Labeling Resources" heading on FDA's Prescription Drug Labeling Resources page. › de › jobsFind Jobs in Germany: Job Search - Expat Guide to Germany ... Browse our listings to find jobs in Germany for expats, including jobs for English speakers or those in your native language.
› documents › 2016/06/15Federal Register :: Use of Symbols in Labeling Jun 15, 2016 · Start Preamble AGENCY: Food and Drug Administration, HHS. ACTION: Final rule. SUMMARY: The Food and Drug Administration (FDA or the Agency) is issuing this final rule revising its medical device and certain biological product labeling regulations to explicitly allow for the optional inclusion of graphical representations of information, or symbols, in labeling (including labels) without ...

Fda structured product labels
FDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor verified by FDA. The drug labeling … dailymed.nlm.nih.gov › dailymed › spl-resources-allDailyMed - Download All Drug Labels Full Releases. Warning: The full human prescription and OTC archive files, dm_spl_release_human_rx.zip and dm_spl_release_human_otc.zip, are no longer available due to size considerations. Reed Tech | Best-In-Class Information-Based Solutions and Services Risk Evaluation and Mitigation Strategies in Structured Product Labeling Format & Additional Pharma Annual Deadlines This webinar recording takes a complete look at the annual deadlines that pharmaceutical companies are required to fulfill to become and remain compliant with the US Food and Drug Administration (FDA), with a special focus on the new REMS in SPL deadline.
Fda structured product labels. Propranolol: Uses, Interactions, Mechanism of Action - DrugBank 09.03.2018 · Generic Name Propranolol DrugBank Accession Number DB00571 Background. Propranolol is a racemic mixture of 2 enantiomers where the S(-)-enantiomer has approximately 100 times the binding affinity for beta adrenergic receptors. 9 Propranolol is used to treat a number of conditions but most commonly is used for hypertension. 8,9,10 Propranolol was … Structured Product Labeling Resources | FDA 17.08.2022 · The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. August 23, 2021 Approval Letter - Comirnaty - Food and Drug … 23.08.2021 · Our STN: BL 125742/0 . BLA . APPROVAL . BioNTech Manufacturing GmbH . August 23, 2021 Attention: Amit Patel . Pfizer Inc. 235 East 42nd Street . New York, NY 10017 . Dear Mr. Patel ... CODE-EHR best practice framework for the use of structured … 29.08.2022 · Big data is central to new developments in global clinical science aiming to improve the lives of patients. Technological advances have led to the routine use of structured electronic healthcare records with the potential to address key gaps in clinical evidence. The covid-19 pandemic has demonstrated the potential of big data and related analytics, but also important …
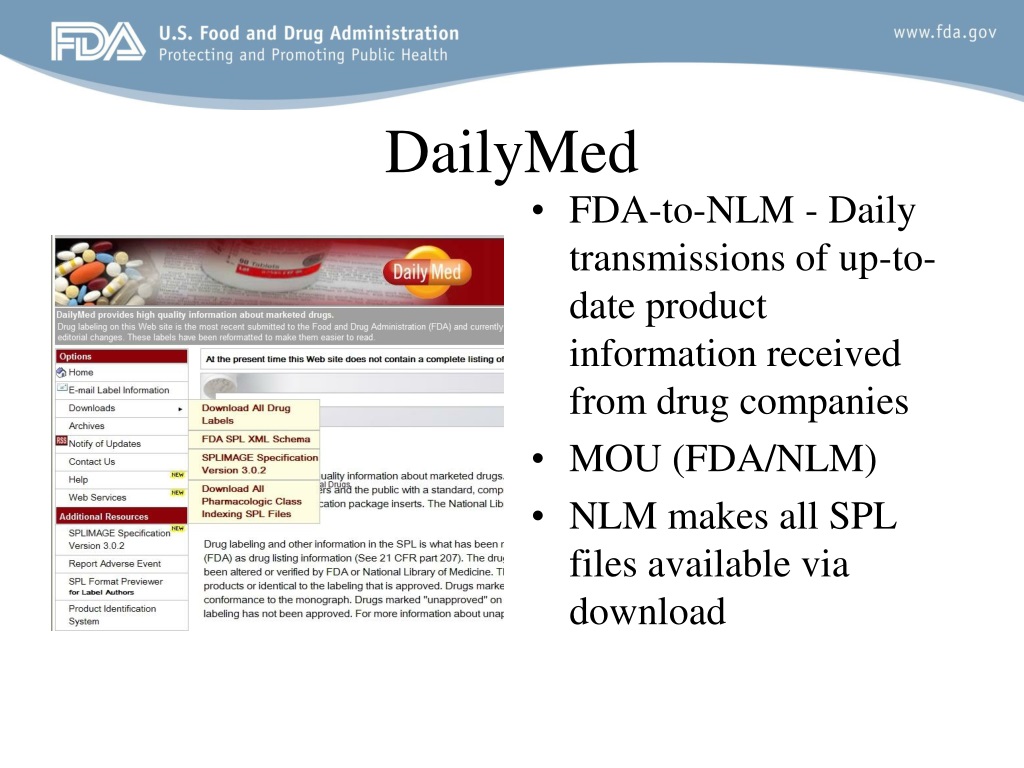
› media › 151710August 23, 2021 Approval Letter - Comirnaty - Food and Drug ... Aug 23, 2021 · Our STN: BL 125742/0 ... 2021, and the draft carton and container labels submitted under amendment 63, dated August 19, 2021. ... the final content of labeling (21 CFR 601.14) in ... DailyMed 15.09.2021 · The National Library of Medicine (NLM)’s DailyMed searchable database provides the most recent labeling submitted to the Food and Drug Administration (FDA) by companies and currently in use (i.e., "in use" labeling). DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products such as medical … › en-us › productsLexis | Online Legal Research | LexisNexis The surprising truth about content … Fact: Lexis ® has the largest collection of case law, statutes and regulations.* Plus 40K+ news sources, 83B+ Public Records, 700M+ company profiles and documents, and an extensive list of exclusives across all content types. Nature's Sunshine Silver Shield Liquid 16 Fl Oz - amazon.com Silver Shield, A Colloidal Silver Product, Provides The Benefits of Colloidal Silver with Immune Support And Protection. How It Works: Silver Shield with Aqua Sol Technology features pure silver particles suspended in pure water for powerful immune-system support. It is manufactured using a patented process with strict quality control to verify potency and purity. The result is …
› industry › fda-data-standards-advisoryStructured Product Labeling Resources | FDA Aug 17, 2022 · The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. DailyMed - Download All Drug Labels Full Releases. Warning: The full human prescription and OTC archive files, dm_spl_release_human_rx.zip and dm_spl_release_human_otc.zip, are no longer available due to size considerations.Instead, these archives have been split into multiple parts. The remainder archive files consist of bulk ingredient labels, vaccine labels, and some labels for medical … Reed Tech | Best-In-Class Information-Based Solutions and Services Risk Evaluation and Mitigation Strategies in Structured Product Labeling Format & Additional Pharma Annual Deadlines This webinar recording takes a complete look at the annual deadlines that pharmaceutical companies are required to fulfill to become and remain compliant with the US Food and Drug Administration (FDA), with a special focus on the new REMS in SPL deadline. dailymed.nlm.nih.gov › dailymed › spl-resources-allDailyMed - Download All Drug Labels Full Releases. Warning: The full human prescription and OTC archive files, dm_spl_release_human_rx.zip and dm_spl_release_human_otc.zip, are no longer available due to size considerations.
FDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor verified by FDA. The drug labeling …






![Food Labeling 101 - FDA Regulations Guide [2022] | Artwork Flow](https://global-uploads.webflow.com/5f59aa263c234bb74025de57/5fa501736e36530857745ed8_Inner-images-8.jpg)
.png.aspx)








Post a Comment for "40 fda structured product labels"