41 fda misleading food labels
The trouble with SPF | EWG's Guide to Sunscreens The FDA has long contended that SPF higher than 50 is “inherently misleading” (FDA 2007). SPF values are limited to 50+ in most countires. In 2011, the FDA proposed prohibiting labels higher than SPF 50+. But in its final draft sunscreen rule, published in 2021, the agency proposed raising the cap to 60+. EWG believes the FDA should ... Cat Feed Inspired Human Food? - Truth about Pet Food Pet food manufacturers would be prevented from misleading pet owners IF pet feed products were properly/truthfully labeled as feed grade. If you've not read it, please read our pet food label recommendations sent to FDA and provide your support by giving FDA your comment. Wishing you and your pet the best - Susan Thixton Pet Food Safety Advocate
Novavax Reports Second Quarter 2022 Financial Results and Operational ... Received Emergency Use Authorization (EUA) from United States (U.S.) Food and Drug Administration (FDA) with label expansion underway, marking first protein-based COVID-19 vaccine available for ...
Fda misleading food labels
Tobacco packaging warning messages - Wikipedia Tobacco package warning messages are warning messages that appear on the packaging of cigarettes and other tobacco products concerning their health effects. They have been implemented in an effort to enhance the public's awareness of the harmful effects of smoking. In general, warnings used in different countries try to emphasize the same messages. Naming, Labeling, and Packaging of Pharmaceuticals - Medscape The process for naming a marketable drug involves five steps: NCE submission and patent application, generic naming, brand naming, FDA review, and final approval. A pharmaceutical company submits ... Tampon safety scare goes viral on TikTok The FDA categorizes titanium dioxide as "Generally Recognized as Safe," but the scare-causing chemical has recently been banned as a food additive in the EU on the basis that experts could not...
Fda misleading food labels. Palm oil labelling | Ethical Consumer These are still hidden under a plethora of misleading synonyms. Spotting palm oil derivatives Much of the palm oil we consume appears in a number of processed forms, 'derivatives' of the oil itself. These 500 or so different substances make up about 60% of global palm oil use. Prescribing Information Resources | FDA - U.S. Food and Drug Administration The Prescribing Information (PI) has two formats: "Physician Labeling Rule" (PLR) format and "old" (non-PLR) format. Given that all new human prescription drugs approved since June 2001 and certain human prescription drugs approved before June 2001 (e.g., those approved for new uses after June 2001) must have PI in PLR format, this webpage focuses on providing resources for the ... › foodlaw › safe-408-608Regulation of the U.S. Food Processing Sector — Food Law USDA pre-approves labels, FDA does not. For products regulated by FDA, the food business does its best to develop a label but it is only after it has begun to use the label that the business will learn whether FDA considers the label adequate. FSIS, as part of its continuous inspection, must approve a label before it can be used.. journalofethics.ama-assn.org › article › how-fdaHow FDA Failures Contributed to the Opioid Crisis | Journal ... To finally end the opioid crisis, the FDA must enforce the Food, Drug, and Cosmetic Act, and it must act on recommendations from the NAS for an overhaul of its opioid approval and removal policies. The broad indication on opioid labels must be narrowed, and an explicit warning against long-term use and high-dose prescribing should be added.
The Ultimate Guide to False-Advertising Law in New York: 22 FAQs Yes, false advertising under GBL § 350 includes mislabeling. Galaxy Export, Inc. v. Bedford Textile Products, Inc. 443 N.Y.S.2d 439 (2 Dept. 1981). The definition of mislabel is "to label (something) incorrectly or falsely," according to Merriam Webster dictionary. 10) Can the New York Attorney General pursue damages for false advertising? Yes. › food › nutrition-education-resourcesGluten and Food Labeling | FDA Since 2014, the U.S. Food and Drug Administration (FDA) has required that claims on food labels that a food contains no gluten meet a clear standard that assures consumers that “gluten-free ... Azurity Pharmaceuticals, Inc. v. Edge Pharma, LLC, No. 21-1492 (1st Cir ... to prove a lanham act claim for unfair competition and false advertising, a plaintiff must demonstrate that - 8 - (1) the defendant made a false or misleading description of fact or representation of fact in a commercial advertisement about his own or another's product; (2) the misrepresentation is material, in that it is likely to influence the … 'At best, calorie labelling is deeply misleading. At worst, it's ... Food labels don't take into account the calories used digesting said food, which could be between 10 to 15 per cent. Nor do they take into account how many calories your body can actually use. A 1,000 calorie dish will affect every single person who eats it differently.
FDA Infant Formula Update: August 10, 2022 | Morningstar The FDA issued guidance on May 16 that outlined a process by which the agency would not object to the importation of certain infant formula products intended for a foreign market or distribution in... As used in this part: Automatic identification and data capture (AIDC) means any technology that conveys the unique device identifier or the device identifier of a device in a form that can be entered into an electronic patient record or other computer system via an automated process. Center Director means the Director of the Center for Devices and Radiological Health or the Director of the ... Food Labeling & Nutrition | FDA 16.05.2022 · What's new in food labeling and nutrition, including label claims, nutrition labeling for restaurants, and links to industry guidance. Pesticide Labeling Questions & Answers | US EPA 14.10.2021 · If labels do not specifically state that they can be used in food storage facilities or food processing plants, can these rodenticides be used under 21 CFR 110.35(c), which states in part " The use of insecticides or rodenticides is permitted only under precautions and restrictions that will protect against the contamination of food, food-contact surfaces, and food-packaging …
How Chili Crisp Took Over America - yahoo.com FDA chief Robert Califf, however, backed the method saying while the intradermal administration led to some mild-to-moderate side effects, it produced a similar immune response to injecting the ...
Delaware Supreme Court Weighs In On Merger Consideration Split FDA Petitioned for Front-Of-Pack Nutrition Labeling by: Food and Drug Law at Keller and Heckman; ... and the disclosures regarding the scope of appraisal rights were not materially misleading. ...
Insight from company leadership, employees and FDA records provides new ... The FDA labeled the recall as Class II, defined as: "a situation in which use of or exposure to a violative product may cause temporary or medically reversible adverse health consequences or where...

Just Mayo is just not mayo: FDA says eggless mayonnaise must change name | Business | The Guardian
Dietary Supplement Products Marketed for Immune Support Unlike drugs, dietary supplements are not approved by the US government for safety or efficacy. In addition, the US Food and Drug Administration (FDA) does not approve their labeling before going to market. However, the FDA has specific regulations that manufacturers are required to follow for product manufacturing and labeling.
Food Product Dating | Food Safety and Inspection Service 02.10.2019 · 1 The U.S. Food and Drug Administration requires a “use by” date on infant formula.The U.S. Department of Agriculture (USDA) does not require quality or food safety date labels for products under its purview. However, the USDA does require a "pack date" for poultry products and thermally processed, commercially sterile products to help identify product lots …
› food-safety › safe-food-handlingFood Product Dating | Food Safety and Inspection Service Oct 02, 2019 · For meat, poultry, and egg products under the jurisdiction of the Food Safety and Inspection Service (FSIS), dates may be voluntarily applied provided they are labeled in a manner that is truthful and not misleading and in compliance with FSIS regulations.[2] To comply, a calendar date must express both the month and day of the month.
Daily multivitamin may be an unnecessary habit There are some exceptions, however. Highly restrictive diets and gastrointestinal conditions, or certain weight-loss surgeries that cause poor nutrient absorption, are examples of reasons why a ...
Roche announces U.S. FDA approval of Xofluza to treat influenza in ... Hans Trees, PhD Phone: +41 61 687 41 47. Nathalie Altermatt Phone: +41 61 687 43 05. Karsten Kleine Phone: +41 61 682 28 31. Nina Mählitz Phone: +41 79 327 54 74. Dr. Barbara von Schnurbein Phone ...
Import Alerts by Publish Date - Food and Drug Administration detention without physical examination of cat food products due to the presence of propylene glycol: 98-02: dwpe: 07/14/2021: detention without physical examination of regulated tobacco products whose label, labeling, or advertising uses descriptors of light, mild, or low: 54-18: dwpe: 07/09/2021
Beyond Air® Reports Financial Results for the First Quarter of Fiscal ... being conducted by Beyond Cancer™, the Company's oncology affiliate, during the current quarter. Conference call scheduled for 4:30 p.m. ET today, August 11th. GARDEN CITY, N.Y., Aug. 11, 2022 ...
Dairy will lead in nutrition fight - hoards.com FDA's lack of enforcement has allowed misleading food labels to falsely imply nutritional equivalency, causing misinformed consumer choices that harm public health. Dairy also leads efforts to protect choice of nutritious food options in federal programs.
How FDA Failures Contributed to the Opioid Crisis In 2017, the President’s Commission on Combatting Drug Addiction and the Opioid Crisis found that the opioid crisis was caused in part by “inadequate oversight by the Food and Drug Administration,” and the National Academy of Sciences (NAS) publicly called on the FDA to overhaul its opioid policies. 9,10 Last year, a former FDA Commissioner rebuked the agency …
sentientmedia.org › misleading-food-labels13 Misleading Food Label Claims and How Not to Be Tricked Oct 20, 2021 · The Food and Drug Administration (FDA) provides guidelines for a variety of common food labels, including sugar-free. While the term suggests that products labeled this way would be completely free of sugar, they can actually contain up to 0.5 grams of sugar in a single serving size.
FDA: Mole Removers Can Cause "Permanent Injury" — Best Life Products run afoul of this law when they commit "adulteration"—which refers to using potentially dangerous ingredients or contaminants—or "misbranding," referring to when a product is "false or misleading" about its benefits or doesn't include all of the necessary information on its label.
Legislation protects U.S. bison industry, prevents deceptive labeling ... Bennet and Hoeven first introduced this legislation in 2019. In 2018, the senators led a letter to the FDA highlighting concerns with the growing number of imported water buffalo meat and pet food ingredients on the market being deceptively labeled as "buffalo."
Regulation of the U.S. Food Processing Sector — Food Law The U.S. food processing sector is extensively regulated by state and federal agencies. Federal agencies dominate the regulatory oversight: USDA FSIS for the meat and poultry processing businesses and FDA for all other food processing businesses. State agencies also have an active role in overseeing food processing businesses within their respective states, but their role is in …
Complete Lite Foods Calorie Fat Cholesterol And Sodium Counter Copy ... to protect consumers from false and misleading health claims on food labels. A 1987 Food and Drug Administration proposal permitted explicit disease-prevention claims on food labels. This proposal has seriously weakened. FDA's abililty to challenge even what the agency believes are deceptive claims.
Amniotic Fluid / Amniotic Membrane Tissue Audits Based on the November 2017 guidance, the FDA determined that amniotic fluid falls under Section 351 of the Public Health Service (PHS) Act. As such, they would require an approved Biologics License Application prior to marketing. In the absence of such a license, the selling and marketing of amniotic fluid products may be problematic.
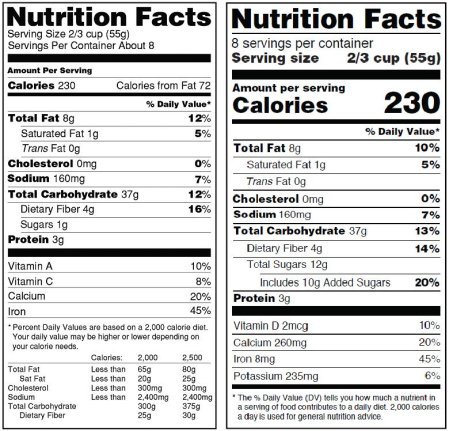


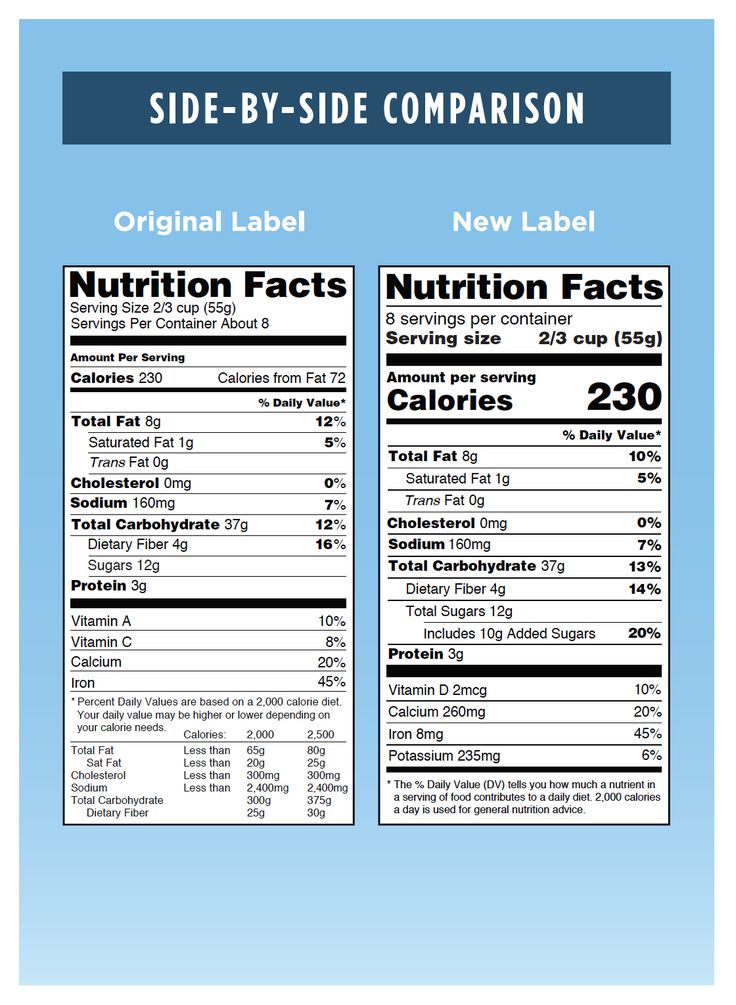
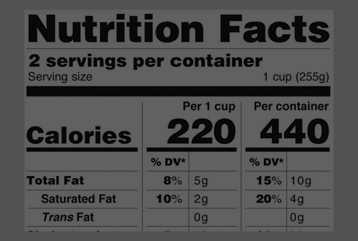






Post a Comment for "41 fda misleading food labels"